Improve your immune system
How can we improve our immune system?
Inside your intestines is a large organ called the “intestinal microbiome”. It is a sensitive organ, made up of billions of microorganisms, actively working to maintain your health and well-being. One of the most important functions of the intestinal microbiome is to work closely with your immune system and therefore it is a good idea to consider how to treat your intestinal microbiome optimally, in order to strengthen the immune system.
Take good care of your gut microbiome
When the intestinal microbiome’s content of microorganisms is in normal balance, which in a fine word is called eubiosis, your body has a good ability to protect itself from external influences.
This is why you can strengthen your immune system by taking good care of your gut microbiome . The key to achieving and maintaining this state lies in both our eating habits and our general lifestyle. The following is good advice on which diet and supplements contain the most important things that the intestinal microbiome must have in order to maintain the fine balance.
Besides vitamins, it is first and foremost prebiotics and probiotics that you need to go for. Although they almost sound the same, they are completely different. You can read about the difference below.
PROBIOTICS AND PREBIOTICS – WHAT’S THE DIFFERENCE?
What are probiotics?
Probiotics are i.a. lactic acid bacteria which are also found in the intestine.
What are prebiotics?
Prebiotics are non-digestible fibers that stimulate the growth of beneficial bacteria in the digestive system.
Go for prebiotics
Prebiotics are a type of fiber that is essential for your intestinal system because they help feed the benign bacteria found in your microbiome. It is also what you may have most often heard referred to as intestinal flora. It is important that these bacteria are fed so that they can be strong and work optimally. Prebiotics are easy to find in normal natural foods, but are also available as supplements.
Foods that contain prebiotics:
• Vegetables: Artichoke, asparagus, beetroot, broccoli, eggplant, leek or mushrooms
• Fruits: Banana, apple, pear, plum, citrus fruits, tomato, plum and sharon fruit
• Whole grains: Oats, rye, barley and spelled
• Legumes: Lentils, chickpeas, split peas, soybeans or dried beans
• Oilseeds: Walnuts, hazelnuts, sunflower and squash seeds
• Spices: Cinnamon, ginger, black pepper or oregano.
Supplement with probiotics
Probiotics are i.a. lactic acid bacteria , which are similar to those found naturally in the intestine. Lactic acid bacteria convert carbohydrates into lactic acid, which is important because it stimulates the development of beneficial microorganisms, or bacteria, in the gut. They also help to ensure that harmful bacteria in the stomach and intestines are weakened. It is therefore a good idea to make sure to get probiotics as part of the daily diet. You can also supplement by eating supplements with probiotics.
Foods that contain probiotics:
• Yogurt
• Fermented milk
• Kefir
• Soy derivatives
• Sauerkraut
Do not forget your vitamins
Your immune system also needs antioxidant vitamins such as vitamins C and D. They are essential for your immune system to function optimally.

Vitamin C
Vitamin C helps to stimulate the cells that inhibit disease-causing bacteria. They also help create proteins that the body needs to form tissues and whole wounds. Unlike most animals, humans are not even able to form vitamin C and therefore it is important to get through the diet.
Foods that are especially rich in vitamin C:
• Fresh parsley
• Citrus fruits
• Blackcurrants
• Kiwi
• Spring salad
D vitamin
Vitamin D helps the body absorb calcium from the intestines, maintain normal bones and teeth and strengthen your immune system. You can get vitamin D through the diet, which, however, will only cover approx. 20% of your need, but the body also forms vitamin D in the skin when exposed to sunlight. Because we in Denmark have limited amounts of sunlight during the winter, the Danish Health and Medicines Authority recommends that you eat a supplement of vitamin D from October to April.
Foods that contain vitamin D:
• Fatty fish (sardines, salmon, herring, mackerel and tuna)
• Eggs
• Cod liver oil
• Butter (less content)
• Meat (less content)
• Milk (less content)
• Cheese (however less content)
When do you need to pay extra attention?
Because your gut microbiome is such an important part of your immune system, it’s always important to pamper it through diet and any supplements and vitamins. But it is also important to know the times when it is good to give it extra attention.
Times for extra attention:
• When your diet is unbalanced (less than 5 fruits and vegetables a day)
• When you have been ill (two infectious episodes during the winter)
• When you have taken antibiotics
• When you are rarely out in the sun
Sources
• Microbiota and intestinal immune system, The basic elements of digestive pathology, CDU-HGE / Editions Elesevier Masson – October 2014. • Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immune system in health and disease. Cell res. 2020; 30 (6): 492-506. • Piqué Nielsen, Berlanga M, Miñana-Galbis D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int J Mol Sci. 2019 May 23; 20 (10): 2534.
Share this content
The impressive forces inside you
The impressive forces in your intestinal system
In your gut there is a whole world where billions of microbes are actively working to maintain your well-being and actually help to influence your mood. That world is, in fact, an organ called the intestinal microbiome – and it plays a vital role in your digestive tract, your immune system and your brain. Therefore, in order to maintain good well-being, it is important to understand the microbiome and know how to best feed it, maintain it, and maintain the fragile balance.
How does your gut microbiome work?
The body itself is a collection of more than 1,000 microorganisms, bacteria and fungi, which together weigh almost 2 kilos and which actively collaborate on the processes that control the body’s functions.
The role of your microbiome is fundamental, as it is first and foremost what makes your body absorb and use the energy provided by the food you eat. It is a big job, which consists both of getting the various nutrients utilized optimally, but also that they are cleaned up afterwards, and that the substances that cannot be absorbed into the body are removed.
Bacteria that will do you good
The bacteria that are part of the microbiome are not pathogenic. It’s a nice word, which really just means that they are not pathogenic – they just want you well. The microbiome consists of a huge number of these “beneficial bacteria”, which have various beneficial functions when combined. Here are some of the most important of these features.
- They help you digest, by participating in the breakdown and excretion of leftover food that you cannot absorb in the small intestine.
- They participate in the cooperation between good molecules, which affect your health, especially by facilitating the absorption of essential nutrients for the body and ensuring the cooperation between certain vitamins.
- They help maintain the intestinal barrier, which is responsible for secreting digestive enzymes and absorbing nutrients. They do this, among other things, by producing fatty acids, which have a positive effect on the mucous membrane, which protects the barrier against the contents of the intestine.
- They strengthen your immune system by limiting the uptake of harmful bacteria and toxins through the barrier, thus preventing unwanted bacteria from multiplying.
The brain and the stomach talk together
The microbiome also includes the very important neurons. No less than 200 million of them. The function of neurons is to communicate with our brain and therefore there is a clear connection between our stomach and our brains. The connection goes both ways, and we probably know it best from when we are worried and have the feeling of having a lump in our stomach. It’s actually just our brain telling the stomach that we’m insecure about something. In the same way, our stomachs can tell the brain when it lacks a specific diet – and therefore the microbiome influences our choice of food via communication with the brain. What few people know is that the microbiome also directly affects our mood. It takes place via the substance called serotonin, which is a substance that is responsible for controlling our emotions.
An intestinal microbiome in balance
Now that we know more about the many important functions of the gut microbiome, it is easier to understand the importance of the microbiome feeling good. This means that we ourselves are well. In order to have a microbiome in balance, one must first and foremost make sure to eat varied. In this way, care is taken to feed the many microorganisms with different types of nutrients and thus maintain a good “culture”.
However, even with a varied diet, you may need to maintain the microbiome by adding lactic acid bacteria , which help maintain balance. There are many products on the market that do just that, but common to many of them is that they are age and need dependent. Therefore, it is important that you make sure to use products that are appropriate for your age.
6 things worth remembering. The microbiome is essential for your health and well-being. In particular, the microbiome ensures:
- Production of vitamins, including B12, B8 and K vitamins.
- Stimulation of the immune system by learning to distinguish allies from enemies.
- Protection against harmful microorganisms.
- The production of important molecules that travel throughout the body.
- The digestion of the nutrients it converts into vital energy for the body.
- The synthesis of 95% of your serotonin – a neurotransmitter responsible for controlling emotions.
Sources:
Série santé 2030 – Emission on the microbiote: a super organism
Intestinal Microbiote (intestinal flora), Inserm.
Jandhyala, SM, Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M., & Nageshwar Reddy, D. (2015). Role of the normal gut microbiota. World Journal of Gastroenterology, 21 (29), 8787–8803.
Health series 2030 – Program on microbiota: a super organism
Intestinal microbiome (intestinal flora), Inserm.
Beneficial Effects of Probiotic Consumption on the Immune System
Introduction
The gastrointestinal tract (GT) is one of the most microbiologically active ecosystems containing a mass of bacteria crucial for the maturation of immune cells. In the gut, a large number of bacteria from the microbiota and those that reach the intestine through food intake, coexist with each other and with the immune cells associated with the lamina propria of the villi. This intestinal microbiota does not interact directly with the epithelial cells; however, the microbiota stimulates the maturation and functionality of the immune cells through their metabolites [1].
There is a group of beneficial bacteria called probiotics. Initially they were defined as “Live microbial feed supplements which beneficially affect the host, improving its intestinal microbial balance” [2]. This definition was revised, and currently probiotics are defined as “Live microorganisms that when being administered in appropriate doses, confer a benefit to the health of the host” [3]. Many probiotic bacteria are members of the intestinal microbiota, some of which have been increasingly incorporated into foods to improve the gut health by maintaining the gastrointestinal microbial balance.
The most common microorganisms used as probiotics are lactic acid bacteria (LAB), particularly the genus: Lactobacilli, Streptococci, Pediococcus, Enterococcus, Bifidobacteria, and some yeast like Saccharomyces boulardii [4]. However, not all the bacteria can be probiotic, as they need to be strain-specific.
The beneficial effects of probiotics have been extensively used in improving the host health and for treating different infectious and non-infectious pathologies in animal models. Namely, protection against infections [5–7], relief of irritable bowel symptoms [8], inhibition of Helicobacter pylori growth [9], prevention of cancer [10–12], decrease in gut inflammatory response [13], and prevention of allergies [14, 15]. In humans, although probiotics have shown encouraging results in several health conditions like diabetes, multi-drug resistant pathogens, irritable bowel syndrome [16–18], exhaustive research is still required to incorporate probiotics into human health, nutrition, and regulation of different abnormalities.
Mechanisms Induced by Probiotics to Stimulate the Immune System
To exhibit beneficial health impact, probiotic microbes should be able to survive in harsh conditions of the stomach and GI tract of humans. This claim may include the ability of the probiotics to withstand the gastric juice and bile salt, survive passage through the upper GT, multiply, colonize, and function in the gut [19]. Many microbes claimed as probiotics could not survive the acidity level of gastric juices and bile salt.
One of the ways probiotics promote human health is by inhibiting the growth of pathogenic bacteria. Probiotics compete for nutrients for growth and proliferation that would otherwise be utilized by pathogens. Several studies demonstrated that probiotics such as Lactobacillus rhamnosus strain GG and L. plantarum showed the ability to inhibit attachment of enteropathogenic Escherichia coli in the GI tract [20].
Additionally, one of the most important properties required for a potential probiotic strain is the capacity of sticking to the epithelial cells. In this regard, Galdeano et al. [21] demonstrated using electronic microscopy that 2 probiotic microorganisms, L. casei CRL 431 and L. paracasei CNCM I-1518, adhere to the intestinal epithelial cells (IECs) through the Toll-like receptors (TLRs) and mediate immune stimulation. Following this interaction, an increase in the cytokines production such as IL-6 and macrophage chemoattractant protein 1 from the IECs occurred, without altering the intestinal barrier; only a slight increase in the mononuclear cell infiltration of small intestine was observed.
The authors also demonstrated that only fragments of the probiotic bacteria, and not the whole bacteria, were internalized inside the IECs. As a consequence, the IECs initiate a complex network of signals that stimulate the immune cells associated with the lamina propria and activate mainly the innate response and the cytokines released by T cells [21].
The intestinal epithelium exhibits numerous physical adaptations to separate the host connective tissue from the external environment. This physical barrier includes a single layer of epithelial cells, their intercellular tight junctions, and the mucus that covers the epithelial surface [22]. Additionally, this physical barrier is reinforced by numerous biochemical adaptations such as a glycocalyx formed by the secretion and apical attachment of a heavily glycosylated mucin-rich layer by Goblet cells. Together, these form a viscous and relatively impermeable sheet on the apical surface of the epithelium [23]. In view of this, probiotics have been shown to strengthen the intestinal barrier by increasing the number of Goblet cells which reinforce the mucus layer [24]. Moreover, several Lactobacillus species have been shown to increase mucin expression in human intestinal cell lines [25, 26]. VSL#3, which contains some Lactobacillus species, increases the expression of MUC2, MUC3, and MUC5AC in HT29 cells [27]. Moreover, L. acidophilus A4 cell extract increased the MUC2 expression in HT29 cells, and this effect was independent of probiotic adhesion to the cell monolayer [28].
One of the ways probiotics promote human health is by inhibiting the growth of pathogenic bacteria through the synthesis of low molecular weight compounds such as organic acid and large molecular weight antimicrobial compounds termed bacteriocins [29]. Organic acids are acetic and lactic acids. These compounds have been proven to exhibit strong inhibitory effects against pathogenic gram-negative bacteria such as H. pylori [30]. Some bacteriocins produced by probiotics are lactacin B from L. acidophilus, bifidocin B produced by Bifidobacterium bifidum NCFB, plantaricin from L. plantarum, and nisin from Lactococcus lactis [31].
Paneth cells, characteristic epithelial cells of the small intestine located at the bottom of the intestinal crypts, are responsible for the secretion of diverse antimicrobial peptides like lysozyme, secretory phospholipase A2, defensins, defensin-like peptides (elafin and SLPI), and cathelicidins [32]. B. longum and a prebiotic (Synergy 1) treatment in patients with active UC provoked the release of defensins from epithelial cells [33]. In addition, the unidirectional peristaltic movements of the intestine also aid in preventing the entry of microbes from the dense distal gut to the small intestine.
Besides, several studies have indicated that probiotics are able to reinforce intestinal barrier integrity through increased gene expression in tight junction signaling. S. thermophilus and L. acidophilus limited adhesion and invasion of enteroinvasive E. coli in HT29 and Caco-2 cells by the maintenance (actin, ZO-1) or enhancement (actinin, occludin) of cytoskeletal and tight junctional protein phosphorylation [34]. Dai et al. [35] showed that VSL#3 probiotics protected the epithelial barrier and increased the tight junction protein expression in vitro and in vivo by activating the p38 and ERK signaling pathways.
Recently, Cazorla et al. [36] observed an increase in Paneth cells at the base of the small intestinal Lieberkühn crypt by the oral administration of probiotics. Accordingly, an increase in the antimicrobial activity of the intestinal fluids that lead to a breakdown of the bacteria was observed by using electronic microscopy. Habil et al. [37] concluded that probiotic strains differentially regulate human beta 2 defensin mRNA expression and protein secretion. These modulations were guided by inflammatory stimulus and cytokine environment.
Few studies reported the bactericidal effect of E. faecium supernatant against an enteroaggregative E. coli, inducing membrane damage and cell lysis [38]. This bacterium has the ability to produce enterocins, which in turn can be applied as food biopreservatives [39, 40].
Antimicrobial peptides could be considered in the future as a new class of therapeutics to induce lesser resistance and have a selective antimicrobial activity to protect the host.
Probiotics modulate the composition of gut microbial species by maintaining the balance and suppressing the growth of potential pathogenic bacteria in the gut. It has been reported that L. acidophilus or L. casei increased LAB with a concomitant decrease of fecal coliforms and anaerobes [41, 42]. In addition, a study by Li et al. [43] found that probiotics caused shifts in the gut microbiota composition toward specific beneficial bacteria, for example, Prevotella and Oscillibacter. These bacteria are known to produce anti-inflammatory metabolites, which subsequently decreased the Th17 polarization and favored the differentiation of anti-inflammatory Treg/Type 1 regulatory T (Tr1) cells in the gut.
A widespread requirement of some probiotic effects is their viability, which means that they must be resistant to acid and bile secretions. In light of this, is the probiotic effect on the gut epithelial cells mediated by particles or by the whole LAB? Do the probiotics have to be viable for immune stimulation? It was demonstrated that only the viable bacteria are able to interact with IECs, and the probiotic cellular fragments are phagocyted by macrophages and dendritic cells (DCs) associated with the Peyer’s patches (PPs) or the lamina propria of the villi. By contrast, non-viable bacteria are cleared fast from the intestinal lumen [21].
How long must these bacteria or their fragments be in contact with the immune cells for their stimulation? To address this question, Galdeano et al. [21] performed an assay using fluorescent probiotic bacteria and analyzed the presence of fluorescence inside the immune cells from PPs, small intestine villi, and lymph nodes of the large intestine. They found that probiotic particles remain until 72 h inside the immune cells, in a similar manner to any particulate antigen. As a consequence of this interaction, probiotics induce an increase in the expression of the receptors TLR2 and mannose (CD206) on the surface of macrophages and DCs. These results reinforce the idea that the main activation induced by probiotics is on the innate immune response [44]. This fact is a key for the later stimulation of an adaptative immune response.
Probiotics confer protection against pathogen colonization by inducing their direct killing, competing with nutrients, and enhancing the response of the gut-associated immune repertoire [45–50].
More important, the probiotic oral administration protects against infection in gut distant mucosas like bronchi and urogenital mucosas [51–53]. A study involving 54 women reported that daily probiotic consumption for 6 months enhanced the clearance of human papillomavirus, which is known to cause cervical cancer [54]. In animal models, oral probiotic administration protects against Salmonella typhimurium infection by activating the phagocytic and microbicidal activity of peritoneal and spleen macrophages [55]. Probiotic lactobacilli can also significantly reduce the risk of antibiotic-associated diarrhea in children and adults [56].
The gut barrier plays a crucial role by spatially compartmentalizing bacteria to the lumen through the production of mucus and secretory immunoglobulin A (sIgA). The IgA antibody is a major functional component of the humoral adaptive immune system, specifically at mucosal sites. The antibodies are predominantly produced by plasma cells localized in the intestinal lamina propria as dimers linked by the connecting chain. The binding of dimeric IgA to the polymeric immunoglobulin receptor contributes to its transportation through IECs and secretion into the intestinal lumen [57]. The secretory component ensures the binding of sIgA to the mucus layer site, where this immunoglobulin leads to the immune exclusion of mucosal antigens [58]. The sIgA has an important role, not only in the gut lumen, but also in the underlying tissue, translocating via M cells, to PP, to preserve the local homeostasis [59–61]. In the intestine, sIgA antibodies bind to commensal and pathogens bacteria, and toxins, blocking them through a non-inflammatory process commonly known as “immune exclusion” [62, 63]. Additionally, sIgA antibodies facilitate the sampling of intestinal environments by DCs in the subepithelial dome region of the PPs. Major efforts are underway to understand the generation, distribution, and maintenance of IgA antibody-secreting plasma cells in intestinal tissues. In this regard, oral administration of probiotics increased the number of IgA+ cells in the lamina propria of the intestine [64] and also in bronchus and mammary glands [13, 65]. These studies demonstrated that probiotics induce the IgA cycle, reinforce, and maintain the immune surveillance in mucosal sites distant from the gut.
T lymphocytes also play an important role in protecting against pathogenic microorganisms in the digestive system, and in regulating the responses against food and commensal antigens. Besides, the adaptive immune system is profoundly shaped by the presence of the commensal intestinal microbiota. This includes increases in the size and number of germinal centers in PPs, IgA-secreting plasma cell numbers, lamina propria CD4+ T cells, and αβ T cell receptor-expressing intraepithelial CD8αβ+ T cells [66]. In healthy mice and humans, the presence of commensal microorganisms in the intestine is tolerated without an acute neutrophils infiltrate. CD4+ regulatory T (Treg) cells are an essential component of this mutualism.
DCs are immune cells with characteristic projections (dendrites), acquired during development, and are specialized for antigen presentation to B and T cells. CD4+ T cells will then differentiate in response to cytokine to different subsets: TH1, TH2, TH17, and regulatory T cells. Probiotic bacteria regulate mucosal immune responses through the induction of different cytokines. This effect is dependent on the probiotic strain itself [67–69]. After oral probiotic administration, cytokines produced by T cells in the lamina propria of the small intestine were secreted in slightly higher levels than those observed in the presence of commensal bacteria; specifically IFN-γ and TNF-α cytokines [70–73]. Through the production of cytokines, probiotics trigger the stimulation of an adaptive immune response and establish a network of signals among the different immune cells. Some probiotics may alter cytokine production by modulating cellular signal transduction. They can either block the degradation of the inhibitor I-κB and interfere with proteasome function, or promote nuclear export of NF-κB subunit RelA, through a PPAR-γ-dependent pathway [74, 75]. IL-10 produced by Th2 lymphocytes and macrophages has been reported to be the main immunomodulator cytokine induced by L. casei CRL 431 to maintain the gut homeostasis [55, 76].
In recent years, there has been an increasing interest in probiotic fermented milk (PFM). Fermentation may improve the digestibility and nutritional quality of food through the enrichment of food substrates like vitamins, proteins, essential amino acids, and essential fatty acids. In this sense, using fermented milk containing probiotic bacteria (PFM), Maldonado Galdeano et al. [77], analyzed the role of the cytokine released by probiotics on immune cells distant from the intestine. The administration of PFM increases the phagocytic and microbicidal activity of the peritoneal and spleen macrophages. Interestingly, probiotics also stimulate the systemic immune response, with an increase in specific antibody production. These antibodies have been shown to play a critical role in decreasing the spread of pathogenic bacteria to the liver and the spleen after a challenge with S. typhimurium. This effect has shown to be more remarkable in an undernourishment model [78].
Malnutrition is a systemic alteration caused by an imbalance between the nutrient intake and energy requirements. It affects the immune response, causing a significant decrease in the defense mechanisms and making the host more susceptible to infections. Hence, malnutrition becomes a good model to study the probiotic impact on the host’s health. On an undernourishment mice model, the administration of PFM as a re-nutrition diet reconstituted the intestinal mucosa architecture and stimulated local and systemic immunity [78]. Considering the fact that malnutrition causes a significant impairment of the immune system, and the thymus being one of the most affected organs, thymus histology restoration by probiotic consumption becomes relevant. The authors also observed a decrease in the cellular apoptosis of this organ and a recovery of the CD4+ and CD8+ single-positive thymocytes. Besides, an increase in different cytokines in the thymus of the mice fed with PFM was also reported [78].
Although information about the minimum effective concentration is still controversial, it is generally accepted that probiotic products should have a minimum concentration of 106 CFU/mL or gram and that a total of 108–109 probiotic microorganisms should be consumed daily [79]. Importantly, the long-term consumption of PFM has been proved to exert immunomodulatory effects to maintain the intestinal homeostasis without secondary effects. The gut immunity balance was preserved and down-regulated by cytokines such as IL-10, avoiding gut inflammatory immune response [80].
The beneficial effect of probiotics in allergy processes is well described [81–83]. The IgE increase is one of the most relevant signs that characterize this process. Probiotics have been shown to be efficient in decreasing this immunoglobulin, as well as in alleviating symptoms. However, the mechanisms mediated for the alleviation of allergy have not been described. In a respiratory allergy experimental model, Velez et al. [14] demonstrated that probiotics induce a clear Th1 balance favoring the production of IgG instead of IgE immunoglobulin and increasing the levels of IL-10 and IFN-γ cytokines. Besides, by a co-localization study, the authors postulate that the Th1 cells have been shown to be responsible for the IFN-γ release.
Furthermore, in vivo studies showed that the administration of probiotics is effective in improving lipid profiles, including the reduction of adipose tissue, serum/plasma total cholesterol, LDL-cholesterol and triglycerides, and increasing the HDL-cholesterol [84, 85]. Clinical trials confirmed that probiotics reduce blood glucose and insulin levels in patients with diabetes. They can also improve Hb1Ac and insulin resistance. Mechanisms for these obesity-related effects include regulation of immune differentiation and insulin sensitivity, inhibition of pathogenic bacteria adhesion to the intestine and translocation to adipose tissue, and improvement of intestinal barrier function [86].
The unquestionable effect of probiotics as anti-cancer agents seems to be due to a combination of multiple mechanisms. Probiotics change the composition and metabolites of the intestinal flora, reduce the number of harmful bacteria, display anti-genotoxic and anti-gene mutation function, and inhibit enzymes in the colon. Besides, through the interaction with colonic cells probiotics regulate the immune system [87]. Probiotics may prevent neoplastic transformation by protecting the mucosal and GT barrier stability, competing with pathogenic bacteria, reducing anti-inflammatory reactions, degrading potential carcinogens, affecting cell proliferation and polyamine metabolism at gastric mucosa [88].
Conclusion
Based on the results obtained, the immune mechanisms elicited by the probiotics are summarized in Figure 1.
Fig. 1.
Immunomodulatory mechanisms exerted by probiotic bacteria in the gut mucosa. Probiotic bacteria adhere to IECs and activate them through the pattern recognition receptors. In this scenario, IECs release cytokines and chemokines that create a microenvironment in the gut lamina propria, bronchi, and mammary glands, allowing the clonal expansion of B cells to produce IgA. At the same time, cytokines stimulated by probiotic bacteria lead to the expression of Treg cells (Foxp3+) that maintain the immune homeostasis in the gut mucosa (unpublished data). PPs macrophages release cytokines after probiotic bacteria stimulation. However, they maintain a characteristic state of hyporesponse to commensal microbiota. Besides, after probiotic stimulation, macrophages distant from the GT such as peritoneum and spleen, increase their functionality (cytokines production, phagocytic and microbicidal activity) reinforcing the innate immune response. Probiotic bacteria administration primes a Th1 profile response, with high levels of IL-10 and IFN-γ that play an important role in the immunomodulation. PP, peyer patch; Treg, T regulatory.

– Probiotics interact with IECs. Due to the privileged position of those cells in the GT, they act as active sensors, setting a dialogue between the host and the external environment. Probiotic bacterial fragments can be internalized into the IECs and produce the subsequent activation of immune cells associated with the gut. This result led to infer that the cell wall of the probiotic bacteria activates the immune system, an activation mediated by TLRs. New studies have been performed with this bacterial structure to confirm this hypothesis.
– Other important cells that play a pivotal role in the epithelial barrier are the Paneth cells. Probiotics have important effects on these cells, increasing their number in the intestinal crypts with the aim of reinforcing the epithelial barrier.
– The time of permanence of the probiotic bacteria in the intestinal lumen (72 h) is enough to induce changes in the gut immune cells, increasing the number of macrophages and DCs of the lamina propria, and enhancing their functionality, reflected in cytokines production.
– Importantly, the activation of immune cells does not alter intestinal homeostasis, probably by the regulatory cells activation that maintains a tolerogenic environment. These facts ensure the safety of probiotics consumption for long periods of time without adverse effects. The cytokine microenvironment generated by immune cells in response to probiotics favors an increase in the gut IgA+ cells. Besides, the cytokines induce locally, influence the activity of immune cells distant from the gut-like macrophages from spleen and peritoneum, and also other mucosa sites such as bronchi and mammary glands.
– In malnutrition processes, the probiotic administration contributes to restore the thymus histology and stimulates the adaptative immune response.
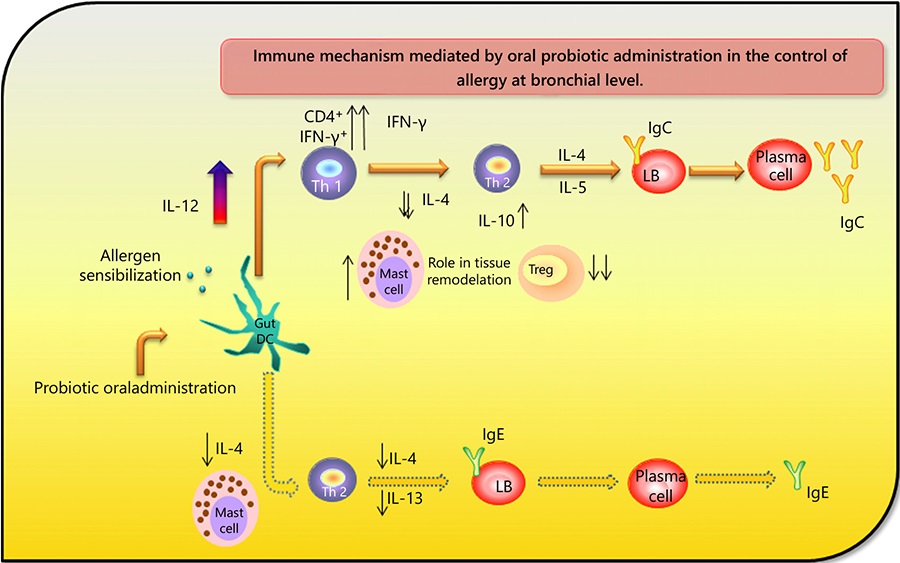
– Probiotics induce a clear balance to a Th1 profile that is essential for the control of an allergy process (Fig. 2).
Fig. 2.
Immune mechanisms mediated by oral probiotic administration to control allergy at bronchial level. The probiotic oral administration induces activation of DCs at the gut level, with an IL-12 release that balances the adaptative response to a Th1 profile at the bronchus. The increase in the expression of CD4 and IFN-γ on Th1 cells leads to an enhancement on IgG production instead of IgE. Treg cells did not increase, so the regulatory effect exerted by the probiotic seemed to be mediated by IL-10, produced by Th1 and Th2 cells. Mast cells are also increased to mediate the tissue repair. In parallel, the Th2 response was significantly diminished with a decrease in the IL-4, IL-13, and IgE production. DC, dendritic cell; Treg, T regulatory.
– Probiotic bacteria, their cell walls or PFM induce signals in the intestine that improve the behavior of the immune system and the host’s health.
– The IECs would be the main target of the probiotics, and together with the innate immune cells associated with the intestine would modulate the mucosal and systemic immunity.
– Probiotic bacteria appeared as an effective tool for the maintenance of the intestinal homeostasis and the stimulation of the mucosal immune system, both at the gut and distant sites.
Acknowledgments
This work was supported by grants from Consejo Nacional de. Investigaciones Científicas y Técnicas (CONICET) Argentina (Dr. Silvia Inés Cazorla, Res 4822) and Dr. Carolina Maldonado Galdeano (PIP 806). Agencia Nacional de Promoción Científica y Tecnológica (PICT 2964).
Disclosure Statement
The authors have no financial conflicts of interest.
| Sources Maldonado Galdeano C.a,b · Cazorla S.I.a,b · Lemme Dumit J.M.a,b · Vélez E.a,b · Perdigón G.a,b Author affiliationsCorresponding Author Keywords: ProbioticsImmune systemMechanismsProbiotic fermented milk |